Abstract
Introduction
Our group previously developed dynamic BH3 profiling (DBP) (Montero el al, 2015), which measures early changes in net pro-apoptotic signaling of mitochondria in response to various drug combinations. The degree of change in the propensity to undergo apoptosis is known as "delta priming". We recently used DBP to demonstrate that BTK inhibition with ibrutinib leads to increased BCL-2 dependence and enhanced sensitivity to the BCL-2 inhibitor venetoclax in CLL cells (Deng et al., 2017). The effects of other novel agents on BCL-2 dependency in CLL cells relative to other anti-apoptotic family members such as MCL-1 and BCL-XL have yet to be systematically explored.
Pathways that are recurrently mutated in CLL represent a logical next area in which to explore the potential of DBP to study the effects of novel agents on the BCL-2 family. One such gene is splicing factor 3B subunit 1 (SF3B1), which is recurrently mutated in a subset of patients with CLL, and confers an adverse prognosis. Here, we treated CLL cells ex vivo with a panel of novel agents including the splice modulator E7107, and utilized DBP to evaluate the functional effects of drug treatments on BCL-2 family proteins.
Methods
Mononuclear cells were isolated from 13 CLL patients and treated ex vivo with E7107 (H3 Biomedicine) and/or one of several other drugs (all obtained commercially). Cells were co-cultured with the human stroma NKTert cell line (1:10 ratio) with 20-hour drug treatments. DBP was performed as previously reported by measuring the release of cytochrome C (cyto-C) in gently permeabilized CLL cells in response to BH3-only peptides using a BD FACS Fortessa. Cyto-C release in response to BAD BH3 peptide was used as a measure of BCL-2 dependence, while cyto-C release in response to HRK and MS-1 BH3 peptide was used as a measure of BCL-XL and MCL-1 dependence, respectively. Western blot analyses were performed by standard protocols with signals captured by an LAS 4000 imager. Statistical analyses were by Welch t-test and one-sample t-test, with a two-tailed nominal p≤0.05 considered as significant.
Results
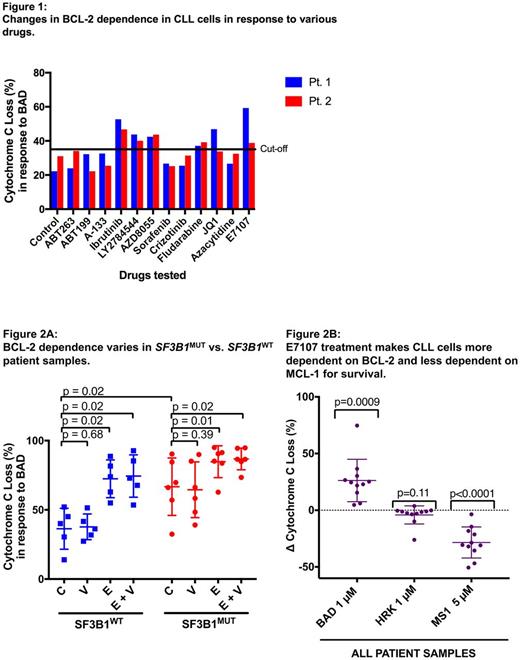
As an initial screen, we treated cells from two different CLL patients in duplicate with a panel of 13 drugs and found that 5 agents resulted in increased BCL-2 dependence (defined as at least 35% cyto C release in both patients) (Fig. 1). These included E7107, ibrutinib, fludarabine, LY2784544 (JAK2 inhibitor), and AZD8055 (AKT inhibitor). As the greatest increase in BCL-2 dependence was observed with E7107, we next explored its effects on 11 additional CLL patient samples (6 SF3B1mut and 5 SF3B1wt). Significantly higher baseline BCL-2 dependence was observed in untreated SF3B1mut cells compared to SF3B1wt (p=0.02) (Fig. 2A). Treatment with E7107 alone or in combination with venetoclax increased BCL-2 dependence of CLL cells irrespective of SF3B1 mutation status (p values between 0.01-0.02). Venetoclax treatment alone had no effect on BCL-2 dependence in either group (p=0.39, p=0.68). In all samples, E7107 treatment made CLL cells significantly more BCL-2 dependent (BAD BH3 peptide, p=0.0009) and less MCL-1 dependent (MS-1 BH3 peptide, p<0.0001), but did not affect BCL-XL dependence (HRK BH3 peptide, p=0.11) (Fig. 2B). Consistent with these findings, CLL cells treated with E7107 had uniformly decreased MCL-1 protein expression on Western blot compared to untreated samples across all patient samples.
Conclusion
We demonstrate the feasibility of using DBP to examine the effects of a panel of novel agents on BCL-2 family anti-apoptotic protein dependence of CLL cells. We identified several candidate drugs to explore in future studies, including the splice modulator E7107, which consistently increased BCL-2 dependence while reciprocally decreasing MCL-1 dependence. These preliminary results support the exploration of DBP in a high throughput format on a larger panel of drugs across more patient samples, with a goal of prioritizing the combinations that appear most promising to explore in clinical trials, to develop more personalized treatment for patients with CLL.
Valentin: Abbvie: Other: Travel reimbursement; Roche: Other: Travel reimbursement. Buonamici: H3 Biomedicine, Inc.: Employment. Neuberg: Synta Pharmaceuticals: Other: Stock shares. Wu: Neon Therapeutics: Consultancy. Letai: AbbVie, AstraZeneca, Novartis: Consultancy, Research Funding. Davids: Merck: Consultancy; InCyte: Membership on an entity's Board of Directors or advisory committees; Infinity: Consultancy, Research Funding; Celgene: Consultancy; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra-Zeneca: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal